As Earth warms, the creatures that spread neglected tropical diseases are gaining a foothold in Europe. Wealthy countries must prepare themselves for more cases.
Europe long thought itself safe from neglected tropical diseases (NTDs). Those old certainties have now evaporated. A warmer and wetter climate has made the continent more welcoming to vectors of debilitating and sometimes deadly pathogens. Climate change is just one of the forces driving the expansion of NTDs. Globalization, and the increase in international trade and travel that it brings, is playing its part in bringing vectors and their pathogens together in Europe. Part of Nature Outlook: Neglected tropical diseases
Although the impact of these diseases is not on the scale of that in tropical countries, the effects on European public health are already being felt. People are catching, and sometimes dying from, NTDs and other mosquito-borne diseases that were once confined to the tropics, such as West Nile, Zika, dengue and chikungunya viruses, as well as parasitic diseases such as schistosomiasis. Cases of vector-borne diseases that are already endemic in Europe, such as leishmaniasis, are on the rise. For many of these infections, there is no vaccine or cure.
Europe is not alone. Parts of the non-tropical world that have previously had the luxury of not worrying about NTDs — including the Gulf Coast of the United States — are experiencing similar issues, says Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas. The globalization of NTDs demands a change in how they are perceived. Historically, NTDs have been largely ignored by funding agencies and left off the health agenda. These diseases affected people living in low-income countries, and supporting action to tackle them was framed as a philanthropic endeavour. But the distinction is becoming blurred as NTDs start to affect high- and middle-income countries. Indeed, Hotez says, it’s now in “the enlightened self-interest of all the G20 countries” to support efforts to combat NTDs.
The new normal
In Europe, the main concern is mosquito-borne viruses. These include West Nile, dengue and chikungunya. (West Nile virus is not officially classified as an NTD, although some scientists think that it should be.) Two decades ago, most cases were imported, but now people are acquiring the viruses locally.
Over the past four decades, Europe has seen the spread of invasive mosquito species such as Aedes albopictus. Originating in the tropical forests of southeast Asia, this insect has spread globally, transported in cargo ships and even in private cars. It was first seen in Albania in 1979, then in Italy in 1990. By 2013, it was established in 8 European Economic Area countries, according to data from the European Centre for Disease Prevention and Control (ECDC), and by 2023 this had increased to 13 (see ‘Mosquitoes move north’). Unlike native European species, it can transmit dengue and chikungunya viruses.
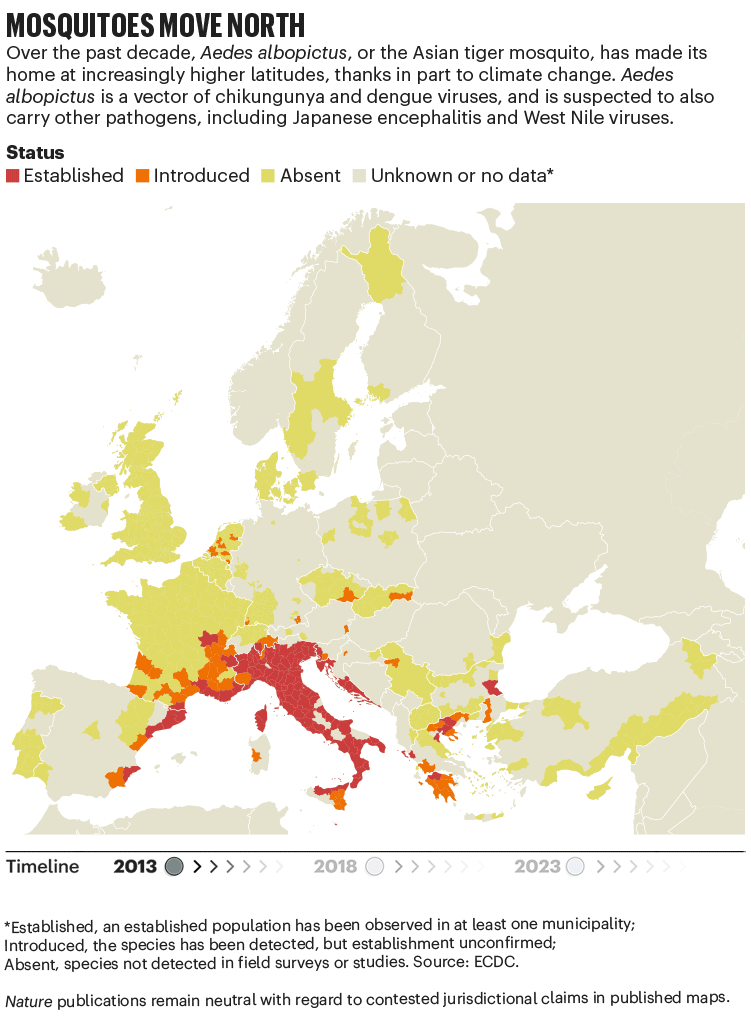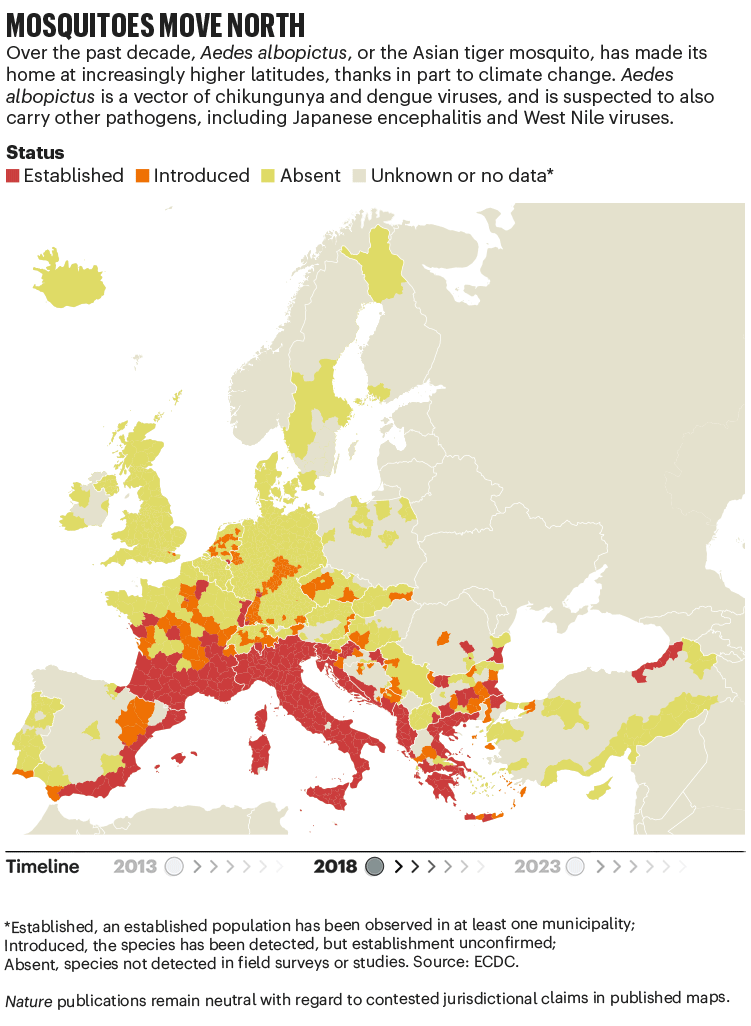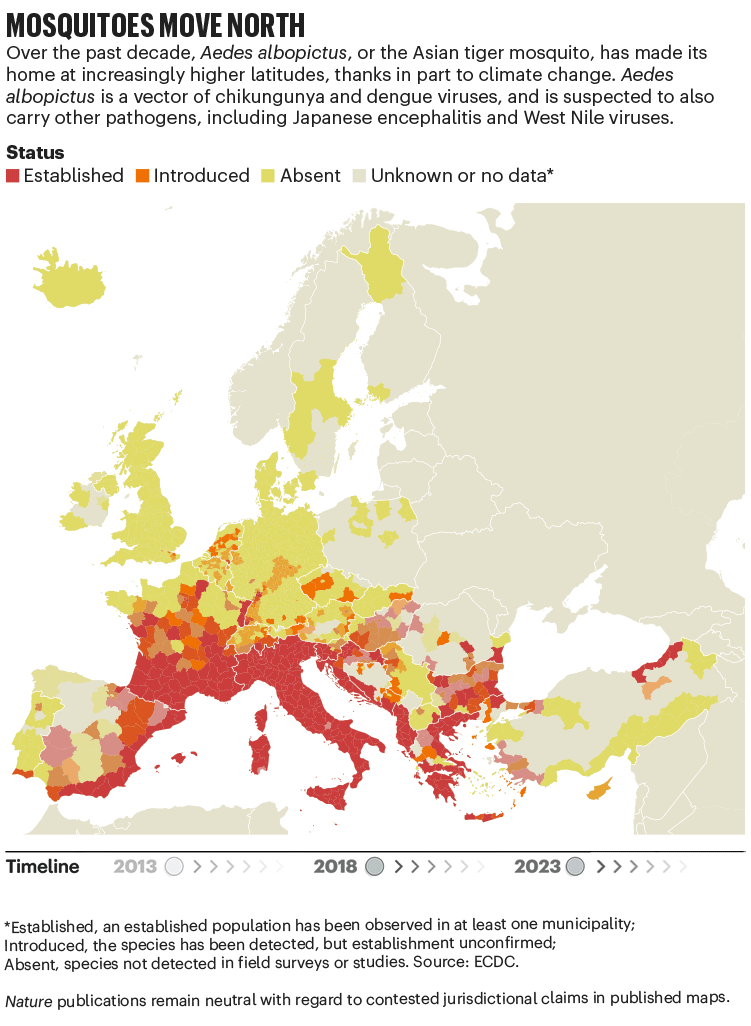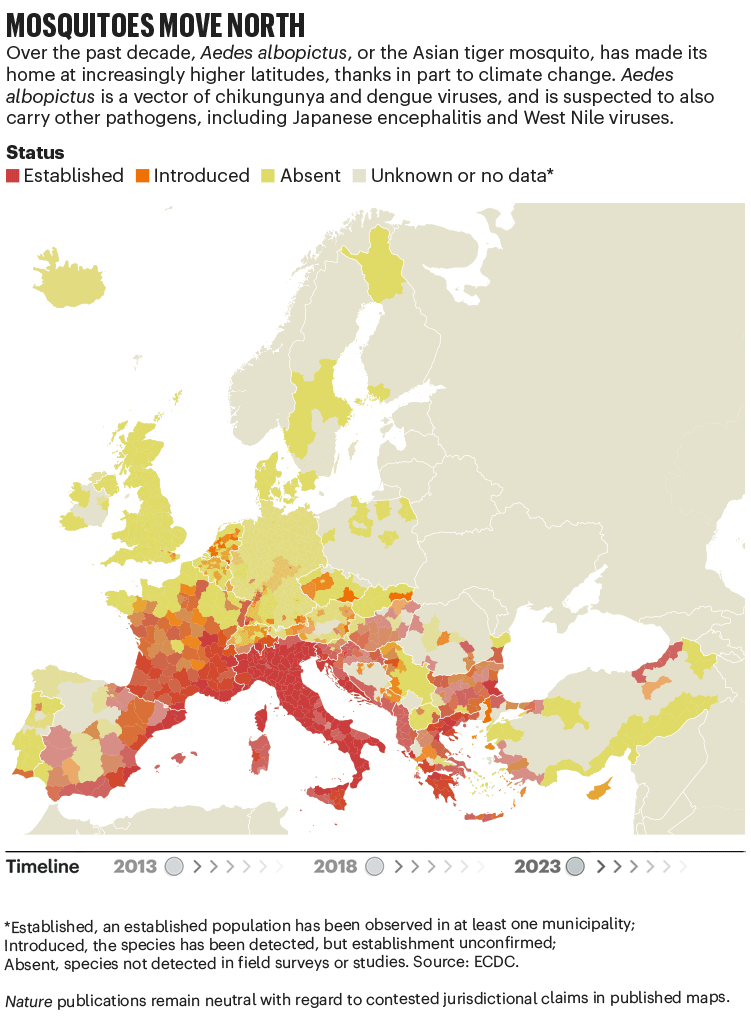
The exact relationship between climate and these viruses is murky. “There’s a lot of unknowns,” says Céline Gossner, who leads a team on emerging food- and vector-borne diseases at the ECDC in Solna, Sweden. But it is clear that climate change has created suitable conditions for the emergence or re-emergence of NTDs. Increasing temperatures, longer summers and flooding are all conditions that suit mosquitoes. Higher temperatures often mean shorter breeding cycles, and faster replication of viruses in vectors, adds Gossner. Temperature change can also alter the migration patterns of birds, which act as reservoirs for West Nile virus.
Climate change also opens the door to species that are sensitive to the cold, such as Aedes aegypti. Originating in Africa, this mosquito spread around the world on ships in the late eighteenth century. It disappeared from southern Europe in the mid-twentieth century, but appeared in Madeira in the early 2000s and was detected in Cyprus in 2022. As well as spreading yellow fever, A. aegypti is more efficient at transmitting dengue, chikungunya and Zika viruses than A. albopictus, and associates more closely with people.

With the vectors in place, viruses arriving in Europe now have a way to spread. Locally acquired dengue cases have been reported since 2010, and numbers are steadily increasing. In 2022, there were 71 cases of locally acquired dengue in mainland Europe, equivalent to the total number recorded between 2010 and 2021. Chikungunya is different, because outbreaks of the virus tend to be more sporadic and intense. There have been two in Europe, one in 2007 and another in 2017.
For the moment, neither disease is endemic; each outbreak is the result of travellers with the disease arriving from abroad. It is possible, says Gossner, that dengue, which had previously disappeared from Europe, could re-establish itself. West Nile virus, however, is endemic. Unlike dengue and chikungunya, West Nile virus is spread by Culex mosquitoes, which are native to Europe. In 2022, there were 1,112 locally acquired cases in 11 countries, the highest since a peak of 1,548 locally acquired cases in 2018. “It’s in most of southern Europe,” says Gossner, “and it’s spreading northwards.”
Need for awareness
These viruses have the potential to become significant threats to public health. Although most cases of West Nile, dengue and chikungunya are either asymptomatic or mild, older people and those who are immunocompromised can die. There are no antiviral therapies. There are two vaccines for dengue, but they are not in widespread use.

One concern is that the diseases could be spread through blood and organ donations. Anyone who has visited an area in Europe that is experiencing a mosquito-borne virus outbreak must, therefore, delay donating blood on their return. But this exacerbates blood-supply shortages that are already a problem in the summer months.
The ECDC receives reports on notifiable diseases from most European countries. This does, however, rely on NTDs being detected — and that’s not a given. People with schistosomiasis, for example, often take several months to develop symptoms. “It’s an insidious disease,” says Jérôme Boissier, who studies the parasitic infection at the University of Perpignan, France. People have to excrete large numbers of eggs in their urine for the infection to be detected in diagnostic tests, he adds.
European physicians need to be alert to the reality that NTDs can be acquired as local infections — and not just during foreign travel. “Part of our job is being watchdogs not only for our patients, but also for public health,” says Camilla Rothe, a clinician specializing in tropical health at the Ludwig Maximilian University of Munich in Germany. In 2019, Rothe and her colleagues identified a case of schistosomiasis in an individual who had returned to Germany after a holiday in Corsica. His case indicated that transmission of the disease in Corsica, which had been thought to be over, was in fact ongoing3.
Physicians must also be aware that diseases that were assumed to affect only animals can and do spill over to people. For example, a number of cases of locally acquired dirofilariasis — caused by an infection with a parasitic worm — have arisen in parts of Europe, including Germany, says Inge Kroidl, a colleague of Rothe’s who studies diseases caused by parasitic worms called helminths. The worms implicated in these cases — Dirofilaria repens — usually infect dogs, but are spread by mosquitoes and can also infect humans. This disease is spreading northwards in Europe, partly as a result of climate change.
Still, many physicians in Europe and the United States are unaware that NTDs can be acquired locally, leading to missed or incorrect diagnoses. Hotez cites an example of a dengue epidemic in Houston that was discovered only retrospectively, when scientists studied clinical specimens. “There were just people coming into the hospital with febrile illness, and nobody thought that maybe this is dengue,” he says. Many think that the new status quo means that NTDs need to be built into the core medical education syllabus. “Tropical medicine is not just a fancy specialization any more,” says Rothe.
Eternal vigilance
For public-health authorities, understanding the risk that these diseases pose depends on knowing where vectors are present and how they are behaving. The ECDC, in collaboration with the European Food Safety Authority, runs a project called VectorNet, which compiles data on the distribution of disease vectors such as mosquitoes, ticks and sand flies across Europe and the Mediterranean region7.

Collecting data such as these, however, is labour intensive, and scientists have to decide where to place traps to collect the insects, says John Palmer, co-director of a citizen-science project called Mosquito Alert in Barcelona, Spain, that encourages people to photograph mosquitoes and report sightings through a dedicated phone app.
Having more eyes on the problem can spot invasive species in unexpected areas. In 2018, for example, a user in Asturias in northern Spain logged the first recorded sighting of Aedes japonicus outside central Europe. This is an example of how citizen surveillance can be a valuable addition to conventional monitoring, says Mosquito Alert co-director Frederic Bartumeus. “Nobody would have been sampling for Aedes japonicus in the north of Spain, he says.” The project also uses traps with an artificial intelligence system to identify mosquitoes on the basis of their characteristic wing-beat frequencies. The system uploads the data to a server every 30 minutes, giving near real-time information on how mosquito populations are growing or shrinking.
This is feeding into models that allow public-health officials to better target their vector-control strategies. Mosquito Alert, for example, is working closely with Barcelona Public Health Agency and has produced a real-time interactive map of A. albopictus activity in the city.
Mosquito Alert aims to expand its reach globally, and work out how to make the app useful in endemic areas where the disease toll is much heavier, says Palmer. Through its partners in a related project called IDAlert — a European-Union-funded project that develops tools to tackle climate-related disease emergence — the team is rolling out the service in Bangladesh, and will soon expand to Vietnam and Brazil. The team is also developing connections in Africa.
Another aim is to develop the app to make it more generally useful for public health, says Bartumeus. This will mean adapting it to function in a context that is very different from that of Europe. For example, malaria is a major concern in Africa, and using the app there would mean monitoring Anopheles species, which transmit the malaria parasite. Anopheles stephensi is a particular concern because this invasive species of mosquito is introducing malaria into crowded urban areas of East Africa — other malaria vectors tend to be confined to rural areas. This is something Mosquito Alert might be able to monitor, Bartumeus says.
Predicting the future
Detailed maps at multiple scales could help scientists to understand the relationships between factors such as climate change and land use, and how mosquito populations respond. These relationships are complex. For example, each mosquito species operates within an optimum temperature range. “There are good examples of infectious diseases now that we fully expect to retreat under climate change because it just gets too hot and mosquitoes can’t survive properly when it exceeds a certain critical temperature,” says Kris Murray, an ecologist at the Medical Research Council Unit The Gambia in Fajara (part of the London School of Hygiene & Tropical Medicine).
So the changes in disease risk will vary, depending on location. “It really depends on what disease you’re talking about, where you are, and even what elevation you’re at,” says Murray. All those variables, he says, “can have a huge impact on the direction of travel for some of these risks”.
It also means that some parts of the tropics will see a decline in vector-borne NTDs. “People sometimes just assume higher temperatures automatically mean more tropical diseases,” says Hotez. “I see it as more of a redistribution.”
Temperature is not the only factor: land use, globalization, inequality and biodiversity all affect NTD vectors, and there is a pressing need to better understand how these all interact. Murray and his colleagues, for example, have been drawing on methods from environmental science, ecology and conservation and combining them with studies of NTDs and their vectors to model mosquito populations.
In one study, they showed how, on a global scale, increasing temperatures are likely to accelerate the potential of Aedes aegyptii to complete its life cycle and advance into areas that it does not currently occupy. In a later study, looking at a smaller scale, they showed how deforestation for palm-oil cultivation in Borneo made the local microclimate more suitable for speeding up the life cycle of Aedes albopticus, which would increase the mosquito population and therefore raise the risk of disease transmission.
Complicating the picture still further is the relationship between the vector and the parasite or pathogen it transmits. If each responds differently to climate change, Murray says, this could trigger a mismatch that applies a selection pressure for the host–pathogen system to evolve an increased disease risk.
Schistosomiasis is a good example of how adaptable pathogens can be. Different species of the parasite can form hybrids, and this can allow it to expand the kind of host it infects as well as the species of snail that act as intermediate hosts, says Boissier. In Corsica, the parasite is a hybrid of a species that infects only humans and a species that infects cattle. Boissier has shown that it seems to infect freshwater snails and mammals more easily than does the species that infects humans.
The details of future trends are yet to be worked out, but the overall trajectory is clear: NTDs are becoming truly global and more must be done not only to understand the complexity of the vector–environment relationship, but also to develop vaccines, diagnostics and treatments for these diseases to help people in all countries. The risks that NTDs pose not only to public health, but also to economic well-being, could finally motivate the governments of rich nations to do more to alleviate the effects of NTDs on people everywhere.
“The other big impact of the neglected tropical diseases is they trap populations in poverty because of their chronic, debilitating effects,” says Hotez. “So these are diseases that hold back economies.” It’s a prospect that the wealthy world can ill afford to ignore. On a hotter, globalized and urbanized planet, NTDs will know no borders.
doi: https://doi.org/10.1038/d41586-023-03476-7
This article is part of Nature Outlook: Neglected tropical diseases, a supplement funded by a grant from Merck Sharp & Dohme and with financial support from Moderna. Nature maintains full independence in all editorial decisions related to the content.